Abstract
Introduction: Despite recent advances in treatment, multiple myeloma (MM) is still considered incurable. Several novel therapies with different mechanisms of action are currently being studied for use in MM. These include targeted therapies such as pathway inhibitors and BCL-2 homology domain 3 (BH3) mimetics. BH3-mimetics overcome apoptosis resistance by binding and inhibiting select pro-survival BCL-2 family proteins. In MM, overexpression of MCL-1 leads to apoptosis resistance and is associated with shorter patient survival. In addition to MCL-1, overexpression of BCL-2 and/or BCL-XL has been observed in MM, suggesting that these three pro-survival proteins are promising targets for therapy. Finding tumor characteristics that can predict responses to MCL-1, BCL-2 or BCL-XL inhibitors is essential for finding optimal therapy combinations for each patient. Besides MM patient age, fitness and disease characteristics, several genetic aberrations are associated with treatment response and patient survival. This study aims at finding predictors of BH3-mimetic inhibitors in MM.
Methods: Using primary MM (n=42) and monoclonal gammopathy of undetermined significance (MGUS) (n=10) bone marrow aspirates and a collection of human myeloma cell lines (HMCL), we determine dependence on MCL-1, BCL-2, and BCL-XL, and investigate whether this correlates to protein expression, disease stage, tumor cytogenetics, and patient characteristics. We use potent and specific BH3-mimetics S63845 (MCL-1), ABT-199/Venetoclax (BCL-2), and A-1155463 (BCL-XL) to assess pro-survival protein dependence. In addition, combinations of these BH3-mimetics are used to determine whether they act in synergy.
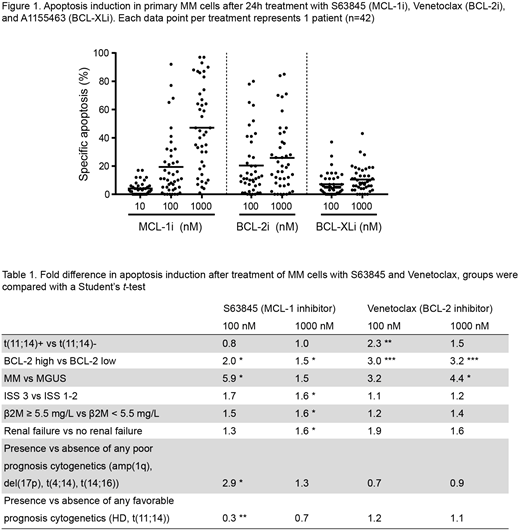
Results: In HMCL, sensitivity to BCL-2 family inhibitors depends to a large extent on expression of BCL-2 and BCL-XL, as was shown previously. Inhibition of MCL-1 efficiently induces apoptosis in HMCL and acts in synergy with BCL-XL inhibition. For primary MM cells, we observed that MCL-1 and BCL-2 inhibitor sensitivity varies greatly between patients. In contrast, MM plasma cells (PC) are generally insensitive to BCL-XL inhibitors as single treatment. As observed with HMCL, combining BCL-2 family inhibitors further increased the level of apoptosis in these cultures. The t(11;14) translocation was previously linked to increased sensitivity towards Venetoclax in MM patients. Our experimental setup and data confirms this correlation (fold difference = 2.3, P=0.009 at 100 nM). Interestingly, we found an even greater difference in Venetoclax sensitivity when comparing patient samples with high versus low BCL-2 protein expression in MM PC (fold difference = 3.0, P=0.0002 at 100 nM). MGUS PC were generally insensitive to BCL-2 and BCL-XL inhibition, and sensitivity to MCL-1 inhibition was limited. These data reveal that MM PC are more dependent on BCL-2 family proteins for survival than PC from premalignant MGUS patients. Next, we investigated whether known poor prognostic characteristics correlate to inhibitor sensitivity and found that MM cells from patients with advanced disease stage (i.e. ISS 3), renal failure, or poor cytogenetics were markedly more sensitive to MCL-1 inhibition (ISS 3: P=0.029, β2M: P=0.020, renal failure: P=0.045, 1q+/17p+/t(4;14)+/t(14;16)+: P=0.039, and HD-/t(11;14)-: P=0.005).
Conclusions: We observed for the first time that cells from MM patients with poor prognosis disease are more sensitive to inhibition of MCL-1. Combining BH3-mimetic inhibitors synergistically increased apoptosis compared to the single inhibitors. In addition, including flow cytometric determination of BCL-2 protein expression in MM cells at diagnosis may aid in predicting patient sensitivity to Venetoclax. Combined, our data indicate that BCL-2 protein expression, in combination with disease characteristics and cytogenetic testing, may predict sensitivity to BCL-2 and MCL-1 inhibitors in subsets of MM patients.
Sonneveld:Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Minnema:Amgen: Consultancy; Celgene: Consultancy, Research Funding; Takeda: Consultancy; Janssen: Consultancy; Servier: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal